Product Overview
Description
The Firegen Viral Molecular Transport Media was designed for the preservation and transportation of viral swab samples. While not containing any guanidine type of inactivating chemicals, it is designed to maintain the integrity of viral DNA or RNA for delayed molecular testing. After heat inactivation of the pathogens, the media can be added directly to the RT-PCR, PCR or isothermal amplification reaction mix without separate viral nucleic acid extraction. This enables a nucleic acid extraction-free RT-RTPCR workflow, and it greatly reduces total turnaround time, lowers costs, and increases throughput.
Specifications
| Product Type | Viral Collection |
| Applications | Viral Collection, PCR, RT-PCR |
| Isolation Method | nucleic acid extraction-free |
| Compatible Sample Types | Nasopharyngeal or Oropharyngeal Swabs |
| Shelf Life | 4 °C for 12 months |
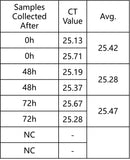
Testing Results of the Preservation System 48 and 72 Hours after Samples Deactivated at 4°C


Note: heat inactivation is required.
Restrictions
- For In Vitro Diagnostic use only in EU regions.
- Research Use Only in the United States.